In this strange new COVID-19 world, everyone is wondering:当这一切将结束?作为lockdowns在不同状态解除,大家都想知道什么时候会真正安全地返回工作,学习,生活的乐趣的活动。
The unsettling reality is that no one knows how long it will take for things to return to normal—and we won’t know until widespread testing is made available to confirm who’s positive out there, says Rashid Chotani, M.D., the vice president of Medical Affairs at CareLife Medical in Fairfax, VA. “Widespread testing is what enables us to discern disease incidence and prevalence, and to determine who has recovered from infection,” explains Dr. Chotani.
World Health Organization (WHO) Director-General Tedros Adhanom Ghebreyesus shares this view. This spring, he tweeted: “One of the main things we've learned in the past months about COVID-19 is that the faster all cases are found, tested, isolated & cared for, the harder we make it for the virus to spread. This principle will save lives & mitigate the economic impact of the pandemic.”
抗体检测可以帮助做到这一点,更。这是因为还有这么多发现关于新的冠状病毒(其只被自去年秋天感染人类)。很多研究人员怀疑,如果它的行为像大多数其他的病毒做的,感染后抗体在我们的血流存在mayprovide some level of immunity—and hopefully allow us to return to our regular lives.
What Are Antibodies, Anyway?
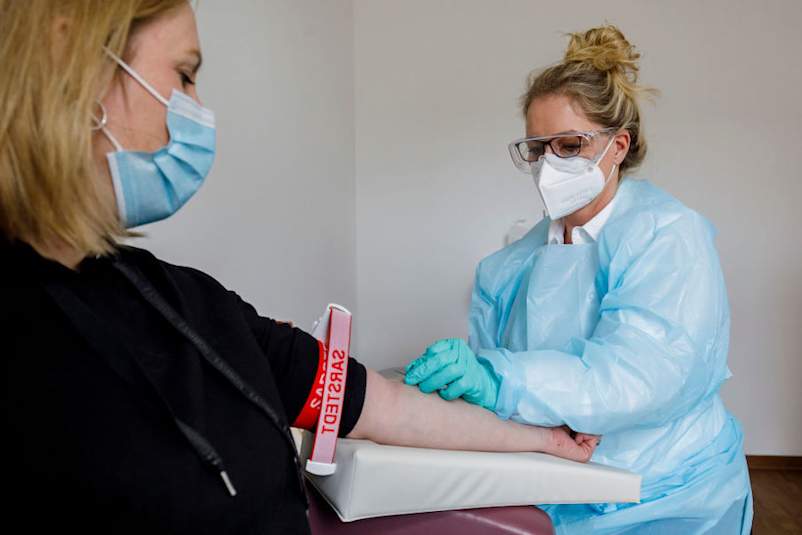
当你发现一个病毒,你的身体会马上开始工作,以抵消它。你的白血细胞产生蛋白质称为抗体反应的感染,其主要工作是踢这个“入侵者”出你的系统。“抗体的存在表明有人已经暴露于病原体在某些时候,”约翰·施密茨,博士,在UNC医院教堂山,NC的临床微生物学/免疫学实验室的副主任说。
两种类型的特定抗体的是COVID-19的重要指标。(它们响应于各种不同的感染冠状的产生仅是一个示例。)
- IgM(short for “immunoglobulin M”) indicates that someone was recently exposed or is currently fighting off an active infection.
- IgG(you guessed it–“immunoglobulin G”) develops later on, and it shows that you’ve developed an immune response to the virus. Whereas IgM peaks and fades within a matter of weeks, IgG can sometimes last for months or even years to provide some level of protection from re-infection.
保护这些抗体提供的水平取决于病毒特异性和COVID-19,科学家们仍然无法确定是否免疫力开发这种方式。但希望的是,这些抗体可能表明至少有一些级别的保护对COVID-19,一旦有人从疾病中康复。
最重要的词有威力。4月17日,世卫组织突发公共卫生事件计划署执行主任迈克尔·瑞安,医学博士警告说,“没有人知道是否有人对抗体具有疾病或发生再次暴露得到充分的保护。”科学家和卫生官员希望知道更多的在未来几个月内。
为什么抗体测试很重要?
In order to know if you have the antibodies for COVID-19, you need a test for them. Unlike polymerase chain reaction (PCR) nasal-swab testing, which tells you if you currently have COVID-19, antibody tests have the potential to tell us who has already recovered from the virus—even if such individuals never showed any symptoms, which occurs in at least 25% of all cases, according to the Centers for Disease Control and Prevention (CDC). This is especially important for us to learn to help stop community spread among people whose symptoms are so mild they might not know they have COVID-19 in the first place—but are continuing to engage with others, potentially transmitting the disease.
This is a really big deal. “The faster we can clear individuals from active disease state, the faster we can mitigate the socio-economic downturn and social disruption,“ explains Dr. Chotani. In other words, the more people we can test, the more experts will know about when it’s safe to reopen the economy again.
How Is the Antibody Test Done?
Karen Dukess, 57, is an author and mom of two living in New Rochelle, NY. She developed suspicious symptoms in early March, headed to Greenwich Hospital in Connecticut (under the instructions of her doctor) to do a drive-through test, and found out她是积极的COVID-19on March 10.
幸运的是,Dukess恢复在家里几个星期内。然后,她立即签署了得到她的血液在西奈山医院在曼哈顿抗体检测。她到达工厂于4月2日,她被赋予了面具,并获得在门口的温度检查。“当时有很多人在那里,两名等候室,”她回忆,描述了人们是如何刻意保持在彼此相距一定距离。
Dukess让她抽血,做了PCR检测,以确保她不再有活动性感染。两天后,她被告知,她确实有抗抗体COVID-19。但她吃惊的是,她还通过鼻拭子检测阳性再次为COVID-19,即使她不再感到恶心。一个星期后,于4月10日,她回到了西奈山的最后鼻拭子测试,以确认病毒不再出现在她的RNA,最后,她为阴性。其中清完下一步:捐献血浆她。
What Is Convalescent Plasma, and Why Might It Help?
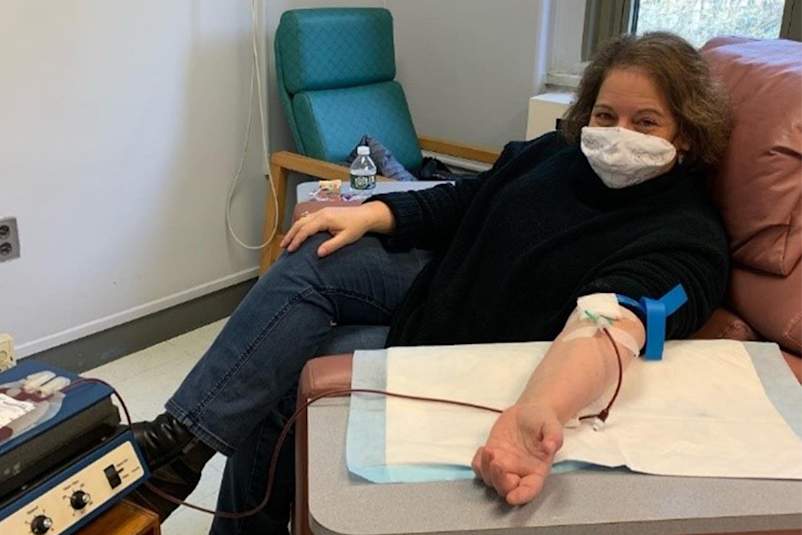
Plasma is the medical term for the golden-colored part of blood containing antibodies, and it’s important because scientists can use it to study the COVID-19 resistant antibodies from recovered patients. Again, at this time it’s unclear whether antibodies indicate immunity—and that’s what scientists are working around the clock to find out. Since such antibodies may hold the key to ending the coronavirus pandemic, survivors are being asked to donate blood or plasma for research purposes.
Whats more, the FDA is encouraging those who have recovered from COVID-19 to donate convalescent plasma in the hopes that it can be used to treat seriously ill patients, by reinjecting it into their bloodstreams to boost their immune response. When you donate plasma, a healthcare provider will draw your blood, put it into a machine to separate out the plasma, then reinject the rest of the blood back into your arm. This is still an experimental approach in the clinical trials stage, but it has been used successfully during other epidemics such as SARS, MERS, and H1N1.
Dukess is hoping to donate her plasma at Mount Sinai, but she’s still waiting to hear back for follow-up instructions. In the meantime, she responded to a request for blood donors from Rockefeller University in Manhattan to participate in a series of studies on prevention and treatment of the virus. Researchers at Rockefeller are looking for “elite neutralizers,” a fancy term for antibodies so resistant to COVID-19 that they could be used to make a drug. In other words, scientists believe that a small group of people may have battled the virus so effectively that their antibodies could help create a cure.
On April 17th, Dukess arrived at Rockefeller to donate blood. The campus was nearly empty. The nurses took her vitals, did a finger-prick hemoglobin test, and then drew about half a bag of blood. “It was frankly one of the most exciting days I’ve had in weeks, because I got to go somewhere and see so many people,” she says. “It felt good to do something useful.” She hopes her early experience with the virus can ultimately help keep other people safe.
我可以成为一个抗体捐赠?
If you’re a recovered COVID-19 patient (or you believe you may have had the virus), you may be able todonate blood or plasma与研究工作的帮助。FDA的网站上提供了有关在那里你可以捐赠信息,并从那里,您可以联系您所在地区的代表。
新建,抗体检测和实验性的治疗举办一些承诺。“来识别个人谁是IgM抗体阴性和IgG阳性是至关重要的,如果我们要恢复到正常一些,并打消劳动力流动性的能力,” Chotani博士说。施密茨队在UNC目前正在找出哪些抗体测试的模型是最准确的,并能以高音量使用。
Should I Get an Antibody Test?
由于抗体测试已成为医院和步入式诊所广泛使用,甚至一些一线雇主(如杂货店)提供的,你可能会急于找到了,如果你有COVID-19抗体,如果你这样做,无论是表明免疫力。不幸的是,在这一点上,免疫仍然是一个巨大的未知数。医生只是不肯定这种病毒行为就像类似的报告。疾病预防控制中心建议,直到有更多的数据是可用的,任何人都不应改变他们的社会疏远的习惯或使用测试结果做出人生决定(如访问年长的亲戚,说,还是回到办公室)。这是因为,即使有抗体存在,甚至不是科学界,如果免疫力是有这个特定病毒,要不了多久,它可能会持续知道。
不过,也许你想知道如果你已经en exposed to the disease, so you'd like to do a test to find out. There are nearly 200 different brands of antibody tests flooding the market right now, yet only 18 of them have received Emergency Use Approval from the FDA. (See thoseapproved antibody tests here。) Of the 18, Schmitz notes that many are manufactured by companies that have produced highly accurate, if non-COVID-19-related, tests before, which is a good sign. However, others on the short list—not to mention the additional 175 or so test kits out there—were created by companies with no history of producing such tests. And accuracy results have been all over the map, with a documented surge of false results. So, the accuracy of the test you’re getting comes down to the specific brand of test your hospital or clinic is using.
"There is no reason that a test performed by a hospital lab versus a point-of-care site [walk-in clinic] would be inherently different," Schmitz says. "Rather, a difference in accuracy would more likely be due to the specific test used. Hospital-based labs will typically use very sensitive technologies to detect antibodies. A walk-in clinic would be more likely to use a rapid test."
Meaning, if your test is done by a hospital requiring a blood draw, like the test Dukess took, it may be more accurate than a finger-prick test done by a walk-in clinic. "May" being the operative word here, because, again, it all depends on the specific test your hospital uses.
According to the American Medical Association (AMA), most of remaining testsnot当中18 FDA批准的试剂盒依赖于制造商的自我验证,意义,还有用于评估测试的准确度确实没有统一的标准。“这么多的考验迅速早就知道我并不感到惊讶了。在准确度变化很大发展,”施米茨说。“我们不知道有关的制造商都已经上市了测试的质量确定性。”假阳性结果可能会导致你假定你已经有COVID-19,当你有没有,这有可能把你和其他人,危险,你应该最终被暴露在病毒。
The AMA advises against individuals using antibody tests to make any assumptions about their health or futures, a point echoed by Schmitz. “The utility of an antibody test for an individual is very limited,” he says. “Antibody tests should not be used as the sole laboratory test to diagnose SARS-CoV-2 infection.” For now, he explains, antibody tests are best used by researchers to screen plasma donors and to conduct studies about general population exposure. As for individual immunity? Only more consistent and accurate testing, plus the science to show that antibodies actually deliver a degree of protection, can prove you have it.
西奈山抗体测试:西奈山。(2020年)“西乃山开发一个‘端至结束’诊断解COVID-19并入诊断,治疗的选择,以及疾病控制的监视。”mountsinai.org/about/newsroom/2020/mount-sinai-developing-an-end-to-end-diagnostics-solution-for-covid-19-that-incorporates-diagnosis-treatment-selection-and-monitoring-of-disease-course-pr
Mount Sinai FDA Approval:西奈山。(2020年),“西奈山的血液测试,以检测抗COVID-19接收紧急使用授权从美国食品和药物管理局。”mountsinai.org/about/newsroom/2020/mount-sinais-blood-test-to-detect-antibodies-to-covid19-receives-emergency-use-authorization-from-us-food-and-drug-administration-pr
恢复期血浆COVID-19临床试验:Proceedings of the National Academy of Sciences of the United States of America。(2020年)“严重COVID-19的患者在恢复期血浆疗法的有效性。”pnas.org/content/early/2020/04/02/2004168117
FDA Plasma Donation:U.S. Food and Drug Administration. (2020.) “Donate COVID-19 Plasma.”fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/donate-covid-19-plasma
FDA Convalescent Plasma Research: U.S. Food and Drug Administration. (2020.) “Recommendations for Investigational COVID-19 Convalescent Plasma.”fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma
洛克菲勒大学的研究抗体:Rockefeller University. (2020.) “Scientists are using 'elite' antibodies from COVID-19 survivors to develop potent therapies.”rockefeller.edu/news/27956-scientists-using-elite-antibodies-covid-19-survivors-develop-potent-therapies/